Voltage operation in a lead-acid battery
In electrical terms, the solar battery is like a voltage source with a small resistance. The voltage it receives can change over a range that depends on the characteristics of the battery.
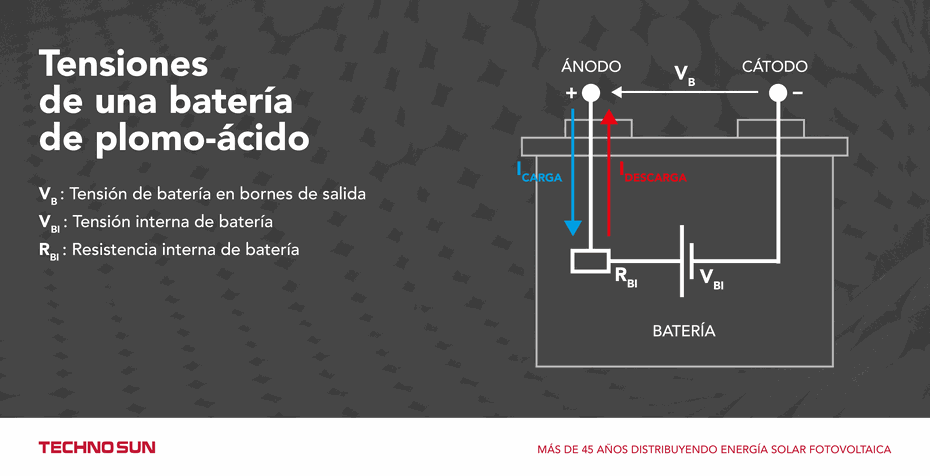
Depending on the temperature of the photovoltaic battery and the concentration of acid in the electrolyte, the value of the internal resistance of the battery (RBI) and the voltage source will vary.
During battery charging, current enters the battery through the anode, which releases acid into the electrolyte causing an increase in its density, and therefore, an increase in VBI and a decrease in RBI due to the higher concentration of ions available for the reactions. On the contrary, during the discharge of the battery the current flows out through the anode producing an absorption of acid from the electrolyte causing a decrease in density, and therefore, a decrease in VBI and an increase in RBI.
When the temperature increases, the water in the electrolyte expands, which leads to a reduction in the acid concentration, resulting in a decrease in the density of the electrolyte and in the value of the electrolyte. VBI. In turn, it increases the fluidity of the electrolyte and the mobility of the ions available for the reaction. The reduction in concentration is compensated for, so that an increase in temperature decreases the value of RBI.
In lead-acid solar batteries there is a direct relationship between the amount of matter and the amount of current generated by the reaction. The voltage of each of the battery elements is about 2 V and their energy density is 170 Wh/kg. When a battery is fully charged, its density is about 1.24 to 1.3 g/cm3.
When a current circulates between the external positive and negative poles and the transport of electrons through the electrolyte. It is then when a series of reversible oxidation/reduction reactions take place causing a transfer of electrons from the electrode to the electrolyte at the positive pole and a transfer of electrons from the electrolyte to the electrode at the negative pole. This discharges the battery causing a decrease in the power difference between terminals according to the intensity of the discharge.
When the voltage applied is higher than 2 V, then the current flows in the opposite direction and everything described above occurs in the opposite direction, thus charging the battery and increasing the potential difference between terminals.
It must be taken into account that the charge voltage decreases with temperature. When the temperature is between 10 and 30 ºC there is no need to vary the float voltage, but if it is outside this range it will have to be adapted with a correction factor of -0.004 V/ºK per element. When the temperature exceeds 40 ºC, the correction factor will be -0.003 V/ºK per element.
State of charge of lead-acid batteries
The voltage between terminals is useful to know the state of charge (SOC) of lead-acid batteries.
The state of charge is the instantaneous capacity of the battery shown as a percentage of the rated capacity.
It is not easy to determine the state of charge because it cannot be determined by simple voltage measurements, but pH levels in the electrolyte is a good way to know the state of charge, although it is not always easy to access the battery electrolyte.
Solar battery voltage values
In a battery bank, voltages are normally 12 V, 24 V or 48 V, although other voltage values can be found more and more in the installations. Some manufacturers work with voltage of 36 V, and when there are high powers it is usual to work with voltages of 120 V or more. With lithium batteries the battery voltage used in isolated systems can exceed 300 V, depending on the battery manufacturer.
Tensiones de una batería de plomo-ácido